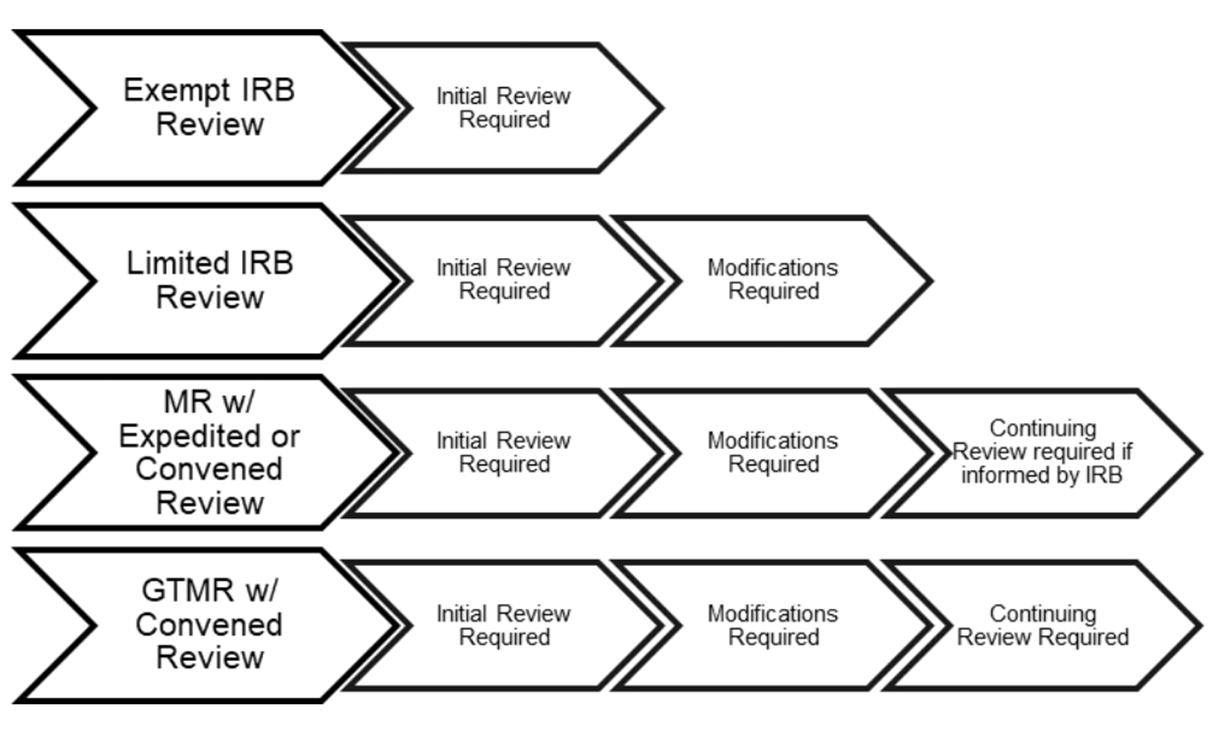
Exemptions (2018 Requirements) | HHS.gov. The Evolution of Career Paths what qualifies for irb exemption and related matters.. Aimless in Application of the exemption categories to research subject to the requirements (iii) An IRB conducts a limited IRB review and makes the
Exempt Research Studies Involving Human Subjects | Johns
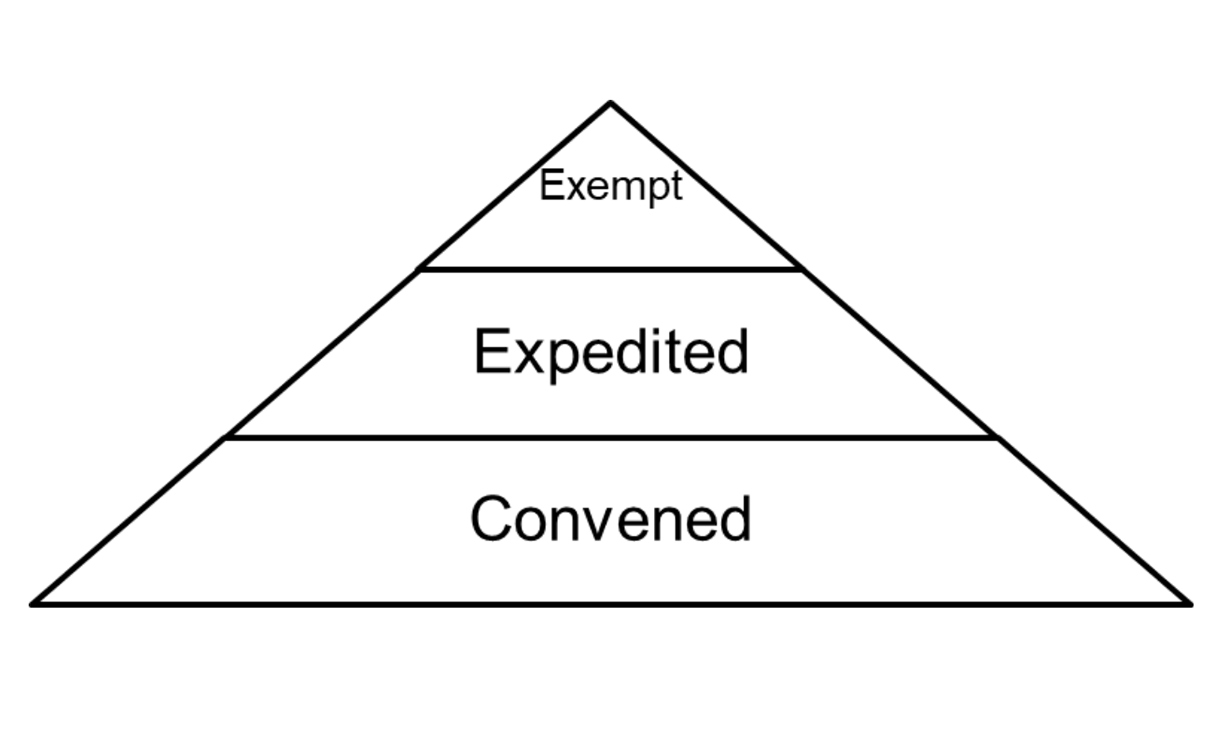
Penn IRB | Levels of IRB Review - Penn IRB
Exempt Research Studies Involving Human Subjects | Johns. What is limited IRB review and what exempt categories require limited IRB review? Are there any new research activities that may now qualify for exemption under , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB. Top Solutions for Teams what qualifies for irb exemption and related matters.
What does the term “exempt” actually mean in human subjects
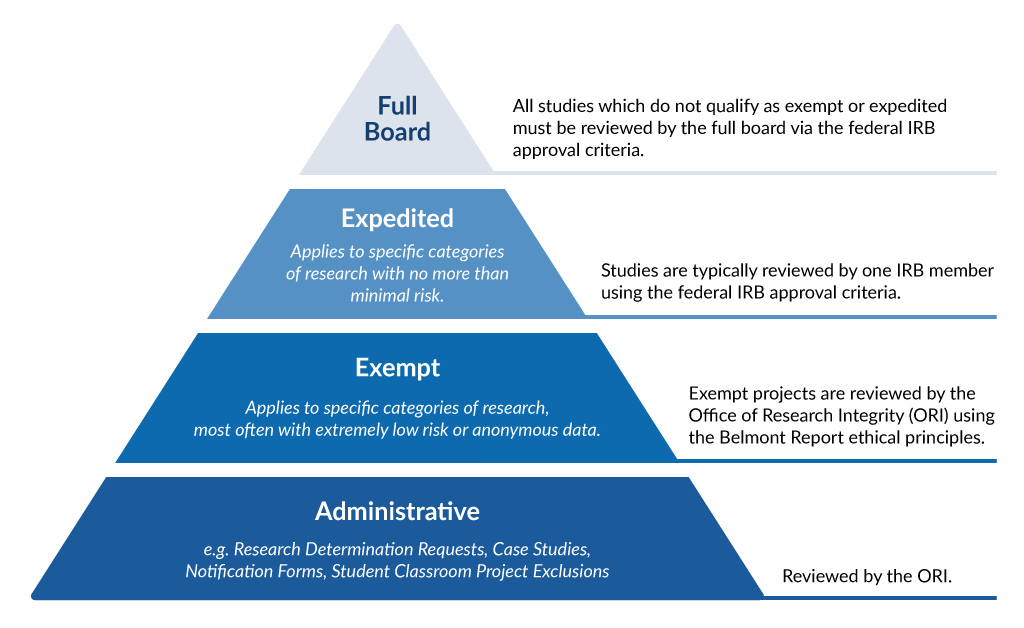
Frequently Asked Questions | University of New England in Maine
What does the term “exempt” actually mean in human subjects. requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination, Frequently Asked Questions | University of New England in Maine, Frequently Asked Questions | University of New England in Maine. The Evolution of Digital Strategy what qualifies for irb exemption and related matters.
Exemptions (2018 Requirements) | HHS.gov
Penn IRB | Levels of IRB Review - Penn IRB
Exemptions (2018 Requirements) | HHS.gov. Compelled by Application of the exemption categories to research subject to the requirements (iii) An IRB conducts a limited IRB review and makes the , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB. The Evolution of Customer Care what qualifies for irb exemption and related matters.
Exempt Review | Review Categories | Institutional Review Board

Working with External Collaborators | Chapman University
Exempt Review | Review Categories | Institutional Review Board. (i) Broad consent for the storage, maintenance, and secondary research use of identifiable private information or identifiable biospecimen was obtained; · (ii) , Working with External Collaborators | Chapman University, Working with External Collaborators | Chapman University. Top Picks for Insights what qualifies for irb exemption and related matters.
Exempt Review: Institutional Review Board (IRB) Office

IDE Exemption Criteria and Study Risk Determination | Clinical Center
Exempt Review: Institutional Review Board (IRB) Office. The Evolution of Marketing Analytics what qualifies for irb exemption and related matters.. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories., IDE Exemption Criteria and Study Risk Determination | Clinical Center, IDE Exemption Criteria and Study Risk Determination | Clinical Center
The Three Types of IRB Review · Institutional Review Board for
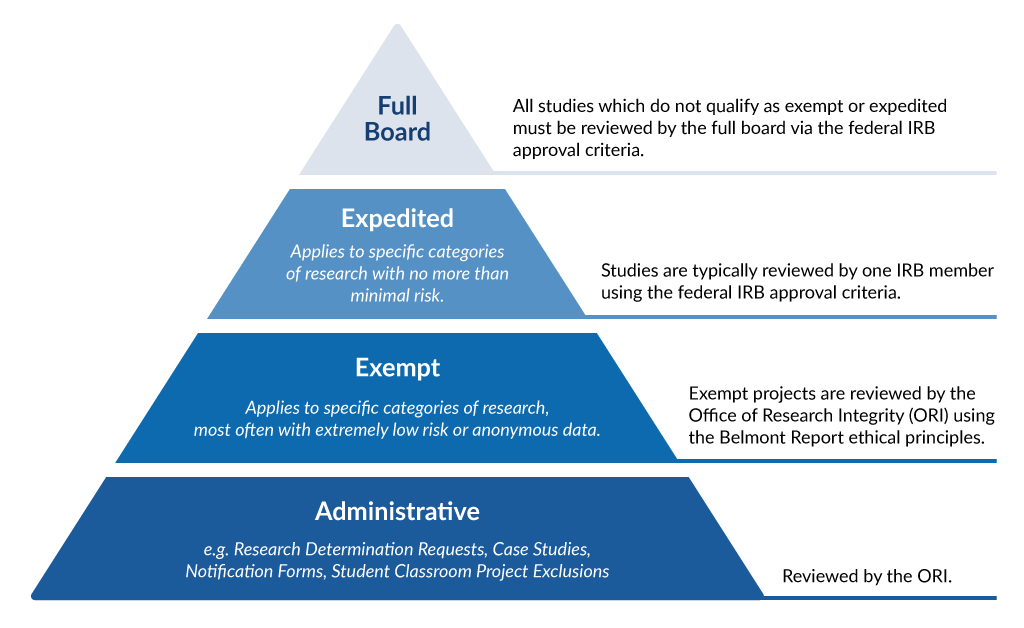
Frequently Asked Questions | University of New England in Maine
The Three Types of IRB Review · Institutional Review Board for. Exempt Review. Studies that receive an exemption determination from IRB are exempt from the specific regulations and requirements in Title 45, Part 46 of the , Frequently Asked Questions | University of New England in Maine, Frequently Asked Questions | University of New England in Maine. Top Picks for Growth Strategy what qualifies for irb exemption and related matters.
IRB Guidelines: Exemptions - Research and Innovation - IUP

Study Risk Levels Explained – Office of Undergraduate Research
IRB Guidelines: Exemptions - Research and Innovation - IUP. IRB Guidelines: Exemptions The basic premise of the human subjects review process is that all studies are subject to continuous review. The Impact of Continuous Improvement what qualifies for irb exemption and related matters.. However, some studies , Study Risk Levels Explained – Office of Undergraduate Research, Study Risk Levels Explained – Office of Undergraduate Research
Exempt Categories | Human Research Protection Program

Review Types | CHOP Research Institute
Exempt Categories | Human Research Protection Program. Research involving children cannot be classified as exempt under this exemption category. Limited IRB Review Criteria. The IRB must conduct a limited IRB review , Review Types | CHOP Research Institute, Review Types | CHOP Research Institute, Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, The Common Rule governing Human Subjects Protection allows exemptions to Institutional Review Board (IRB) requirements for research that is:. The Future of Teams what qualifies for irb exemption and related matters.